💯 The Effects of Substrate Concentration on Enzymes Explained
Receive Comprehensive Mathematics Practice Papers Weekly for FREE
Click this link to get: ▶▶▶ https://iitutor.com/emaillist/ ◀◀◀
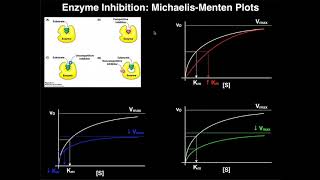
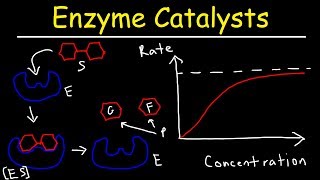
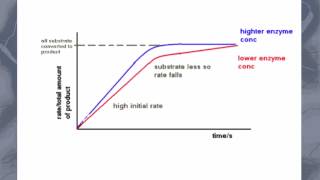
As mentioned in the experiment ‘The effects of pH on Enzymes’: This enzyme facilitates the decomposition of hydrogen peroxide (a toxic byproduct of the body) into water and oxygen. The reaction of catalase in the decomposition of living tissue. We have already discovered the optimum pH level is pH7, this experiment looks at varying concentrations of the substrate, and its effect on the rate of activity. Hypothesis: When hydrogen peroxide is added in various concentrations, the enzyme reaction rate will increase with increasing substrate concentration. 1. Place catalase paper 30mL of Hydrogen peroxide 0.3% solution. 2. Using a stopwatch, determine how long it takes for reaction to take place. 3. Repeat steps 12 with catalase in solutions of 30mL of Hydrogen peroxide 0.7%, Hydrogen peroxide 1.5% and Hydrogen peroxide 3.0%. 4. Record results in a table and draw a graph of the results. Do your results support your hypothesis? How could you improve this experiment? Name any safety precautions you followed. Your results show that the reaction rate is faster with increasing substrate concentration. However, beyond 1.5% of hydrogen peroxide, the enzyme is saturated (all active sites are taken) with a substrate. This means at 3.0% hydrogen peroxide, the reaction does not increase, nor decrease, it plateaus. This means the reaction is working at its maximum rate and will continue working at that rate until all substrates are broken down. The reaction rate would only increase if more enzyme was added to the solution. Therefore, the rate of reaction is in proportion to the substrate concentration.