15 YouTube views, likes subscribers in 10 minutes. Free!
Thermodynamics: ATP hydrolysis problem
00:24 Importance of ATP
01:27 ATP hydrolysis
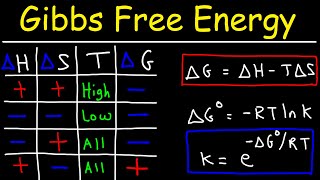
01:56 Assuming ΔG° = 31 kcal / mol
03:01 Relation between equilibrium constant (K) and standard Gibbs free energy (ΔG°)
08:56 Using that in vivo K = 10⁸
11:26 Role of ATP in overcoming Law of Mass Action
Calculation of the equilibrium constant K, assuming that the standard Gibbs energy ΔGº is 31 kcal / mol. Next, the (actual) standard Gibbs energy, accounting for the influence of Mg ⁺², is calculated, using the fact that the actual equilibrium constant K is 10⁸. Finally, the effect of ATP hydrolysis in transforming unfavorable (but necessary) biochemical reactions into thermodynamically favorable ones, is illustrated.
Don't forget to like, comment, share, and subscribe!
Recommended