Type of Reaction for CaCO3 = CaO + CO2
In this video we determine the type of chemical reaction for the equation CaCO3 = CaO + CO2 (Calcium carbonate yields Calcium oxide + Carbon dioxide).
Since we one substance breaking apart into two we have decomposition reaction. Decomposition reactions follow the general form of:
AB → A + B
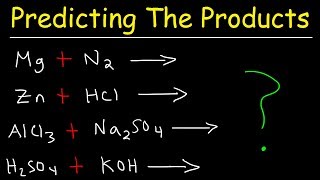
Type of Reactions
Synthesis (Combination): A + B → AB
Decomposition: AB → A + B
Single Replacement : A + BC → B + AC
Double Replacement: AB + CD → AD + BC
Combustion: CxHy + O2 → CO2 + H2O
Neutralization: HX + MOH → MX + H2O
ReductionOxidation (Redox): Electrons are exchanged. Many of the above reactions are redox
Being able to determine the type of reaction by looking at reactants can help in predicting the outcome of the chemical reaction.