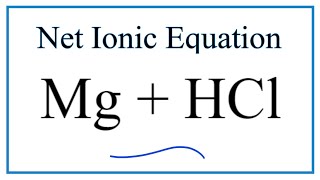Type of Reaction for Mg + HCl = MgCl2 + H2
In this video we determine the type of chemical reaction for the equation Mg + HCl = MgCl2 + H2 (Magnesium + Hydrochloric acid). Since we have a metal replacing another metal the type of reaction is a single displacement reaction (also called a “single replacement” reaction). Single replacement reactions follow the general form of:
A + BC → B + AC
A and B must be either different metals or halogens (Group 17 on the periodic table).
For example, NaBr + Cl2 = NaCl + Br2 or Mg + CuSO4 → MgSO4 + Cu.
To determine if a single displacement reaction will take place, we need to look at the reactivity of the elements involved in an Activity Series table. The table is organized form the least to most reactive. More reactive elements will replace less reactive elements in a single replacement reaction. For this reaction, Mg + HCl = MgCl2 + H2 , the reaction does take place.
Finally, note that all single replacement reactions are also reductionoxidation reactions (redox reactions) as well.
Type of Reactions
Synthesis (Combination): A + B → AB
Decomposition: AB → A + B
Single Replacement : A + BC → B + AC
Double Replacement: AB + CD → AD + BC
Combustion: CxHy + O2 → CO2 + H2O
Neutralization: HX + MOH → MX + H2O
ReductionOxidation (Redox): Electrons are exchanged. Many of the above reactions are redox
Being able to determine the type of reaction by looking at reactants can help in predicting the outcome of the chemical reaction.