Ultimate Guide to the Felkin-Anh Model - Organic Chemistry
The FelkinAnh model in Organic Chemistry explained using a combination of steric factors and molecular orbital interactions (stereoelectronic effects). High diastereoselectivity is achieved for the addition of nucleophiles to aldehydes and ketones that have alpha stereocentres when reactions are performed under kinetic control, with nucleophiles attacking under irreversible reaction conditions.
#chemistry #organicchemistry #orgo #ochem #science #education #stem #stemeducation
Reference:
Total Synthesis of ()Preswinholide A; I Paterson, et al.
J. Am. Chem. Soc. 1994, 116, 6, 2615–2616
https://doi.org/10.1021/ja00085a050
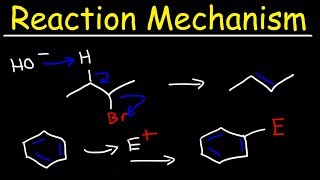
The FelkinAnh model is perhaps the most reliable model as a predictive tool for the observed diastereoselectivity for the addition of nucleophiles to aldehydes and ketones that have a single adjacent (alpha) stereocentre that is composed of three distinct groups – one large, one medium, and one small in size. (Other models to explain diastereoselectivity in such reactions do exist, e.g. the Cornforth model, but are not the subject of this video as over time they have generally proven to be less predictive in general, although really good in specific circumstances.) The most populated conformations of these types of aldehydes/ketones are the ones which orientate the large group perpendicular to the plane of the carbonyl bond. This conformational preference is predominantly controlled by steric effects. When the substrate is in this conformation, one side of the carbonyl is also more blocked by steric effects and so an attacking nucleophile with stereoselectively prefer to react opposite to the large group. Attack of a nucleophile on to a carbonyl occurs via the BurgiDunitz trajectory, which is perpendicular to the plane of the sp2 carbonyl carbon atom, in a plane aligned to the C=O bond, and at a 107 degree angle relative to the C=O bond from the oxygen. This BurgiDunitz trajectory is a compromise between maximising HOMOLUMO overlap of molecular orbitals of the nucleophile with the C=O pi star antibonding molecular orbital and minimising electrostatic repulsion with the filled C=O pi bonding molecular orbital. The most populated conformations project their medium and small groups on the other side the carbonyl. One of these conformers will project the medium group along the direction of the BurgiDunitz trajectory and the other populated conformer will have the small group in that position. Therefore the attacking nucleophile will prefer to attack the carbonyl antibonding LUMO on the flightpath that passes over the small group as a preference as a steric effect. This will lead to the formation of a new stereocentre with good diastereoselectively, often with a diastereomeric ratio of 4:110:1 for reasonably simple substrates, often better with particularly reactive nucleophiles as the reaction can be conducted at lower temperatures and the kinetic control is emphasised. These reactions show the highest levels of diastereoselectivity when there is very good differentiation on sterics between the three groups on the alpha stereocentre to the carbonyl.
When there is an electronegative atom directly attached to the alpha stereocentre, however, there is another stereoelectronic effect that tends to override the above purely sterically based diastereoselectivity. The FelkinAnh model instructs the user to treat the electronegative atom as the large group in the above setup, regardless of the steric size of the group compared to the others present. For the purposes of this discussion, I will use a COMe group, where the C is the alpha carbon to the carbonyl being attacked. As oxygen is much more electronegative than the carbon, the COMe sigma bond is both polarised with electron density being towards the oxygen and also the sigma star antibonding molecular orbital has both a large coefficient on the alpha carbon atom and is relatively low in energy for a carbonbased sigma star MO, by which I mean quite close in energy to the unbonded carbon atom energy. This means that when the COMe sigma star antibonding molecular orbital is arranged perpendicular to the C=O carbonyl bond, it is in fact aligned with the C=O pi star LUMO. Therefore, in this conformations, the two antibonding empty molecular orbitals will combine as they have a similar size and energy match. The combination of these two antibonding molecular orbital results in a new lower energy LUMO for the molecule. Hence in this conformation, the substrate is much more reactive (lower activation energy) than when it is in any other conformation, even if that conformer is not the most populated one.
One final observed effect on diastereoselectivity is observed if the electronegative atom on the alpha stereocentre has available lone pairs (sterically and size). In these cases a fivemembered ring chelate can form if a reasonably strongly Lewis acidic metal cation happens to be present.
.