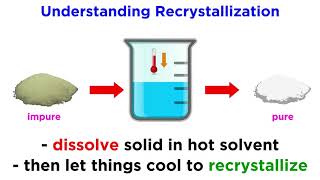
Unsaturated Saturated and Supersaturated Solutions
Solutions may be unsaturated, saturated, or supersaturated, depending on the amount of solute they contain. These categories depend on the solubility of the solute, or how much solute can dissolve in a certain amount of solvent. Solubility is dependent on temperature, and for most solid solutes, the solubility increases as temperature increases. More generally, the amount of solute that a solution can hold is referred to as saturation, so these three types of solutions refer to different amounts of saturation. An unsaturated solution can hold more solute: it has not yet reached its maximum. A saturated solution has reached the maximum amount of solute that it can hold. A supersaturated solution holds more solute than is theoretically possible at a given temperature. More solute can usually be dissolved in a solution if the temperature is raised. If the temperature is lowered, the solute becomes less soluble, and crystals form. This is a process called recrystallization. If you have a saturated solution and you lower the temperature very slowly, and you’re careful not to bump or jar the solution, you can create an unstable situation, which is the supersaturated solution. If a seed crystals is added to a supersaturated solution, it can start the recrystallization process. At the end of the video, we’ll also look at how rock candy is made. Rock candy is sugar crystals on a stick. To make rock candy, you first saturate a solution with sugar at a high temperature. Then, you slowly cool the solution, creating a supersaturated solution. Finally, you add a stick or a piece of string which contains some seed crystals. Recrystallization happens, and you get rock candy: the solid sugar crystals form on the stick.