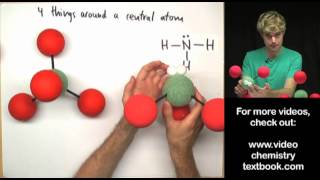
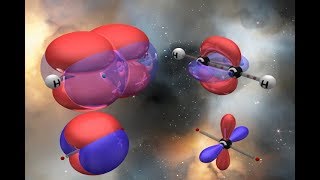
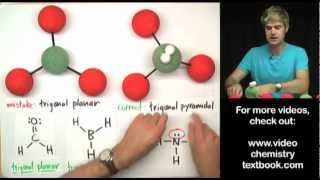
VSEPR: Hybridization Geometries u0026 Bond Angles
Understanding the terminology and geometries of hybridized orbitals (part of the valence bond theory).
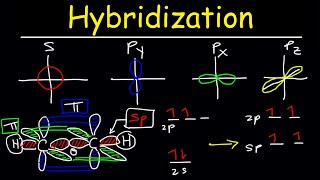
sp = linear = 180 deg
sp2= trigonal planar = 120 deg
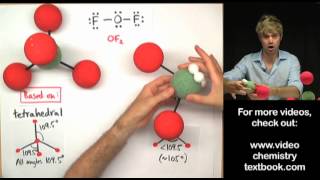
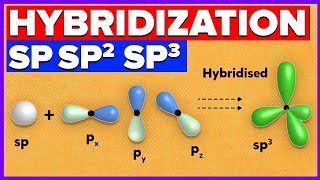
sp3 = tetrahedral = 109.5 deg
dsp3 = trigonal bipyramid = 120 and 90
d2sp3 = octahedral = 90 deg
I also give a brief explanation of sigma and pi bonds, hybridization, and some of the predictions you can make once you've studied the theory.
No, the valence bond theory isn't perfect (consider the paramagnetic properties of O2). It also does a really bad job of predicting some of the real bond angles (measured by crystallography and spectroscopy experimental methods).
Overall, it's still a useful explanation.
...It's helped us understand bonding enough to make predictions about unknown molecules that can be verified by experiment. :) I find this fascinating! :) hehe