Easy way to get 15 free YouTube views, likes and subscribers
VSEPR Theory Part 2: Trigonal Bipyramidal Family
To see all my Chemistry videos, check out
http://socratic.org/chemistry
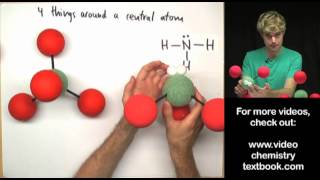
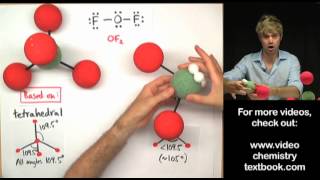
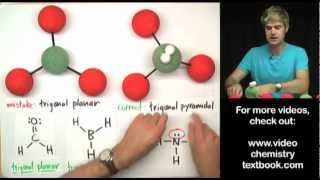
If the central atom in a molecular can make 5 bonds, the structure that it makes is based on the trigonal pyramidal shape. If the molecule has lone pairs or unshared electron pairs, the shape could be see saw, Tshaped, or linear. In this video, we'll look at diagrams of the VSEPR shapes, and examine bond angles for each structure.
Recommended