WCLN - Acid Base Titrations -- Part 1 - Chemistry
The concentration of an acid can be determined by titrating it with a base of known concentration. This video gives an example of such a titration using three separate trials, and it illustrates how the results are used to calculate the concentration of the acid
http://www.BCLearningNetwork.com.
0:03I take creation procedure can be used to find the concentration of an acid
0:07will give you an example and work through the steps in the calculations
0:11a ten milliliter sample ups of uric acid
0:14h2so4 is take trade with .5 Moller Anne awaits addition
0:19a few drops a pheno failing indicator
0:22are added to the acid sample three separate trials are done
0:26rest to find the concentration of h2so4
0:29in the original ten milliliters sample
0:32here's a table showing results of this state region
0:36the initial reading at the beer at is the level up the NaOH
0:39in a beer at before the Tate region is performed in trial 1 a Neoh starts out
0:44at 20 milliliters mark
0:47the final reading at the beer at is the level up the any wage in a beer at
0:51when the equivalence point the tight region is reached in trial 1
0:55this is the point at which the any weight solution reaches the 13point
0:59ninemillimeter mark
1:01now will go back to the results a ball three trials
1:04the volume up any wage used reach trial is calculated by subtracting the initial
1:09reading from the final reading
1:12in trial 1 misses 13.92 leaders
1:150 milliliters which is 13.99 leaders
1:19and try out to the volume used is twenty seven point one milliliters
1:2313 point nine milliliters
1:26which is 13 point two milliliters
1:28and in trial 3 the volume used as forty point one milliliters
1:32minus twenty seven point one milliliters
1:35which comes out to 13.09 leaders
1:39now we need to calculate the best average volume for the any wat used
1:44can see that the value 13.99 for trial one
1:47is considerably larger in the values a 13point to %um 13.04 trials 2&3
1:54respectively
1:56when one guy who is considerably larger or smaller
1:59than the other values we discarded when calculating the best average
2:04so discarding a value 13.99 left with thirteen point two milliliters
2:10and 13.08 milliliters
2:12did establish volume is the average up these two Ballys
2:16which is 13 point two milliliters
2:19plus 13.02 milliliters divided by two
2:23which is 13 point one milliliters
2:26will divide this by 8,000 to convert it to point 0131
2:29leaders have any weight solution
2:33will summarize the information we have been a single statement at the top
2:36the volume a .5 molar Neoh needed to neutralize ten milliliters h2so4
2:44is point 0131 leaders
2:47what were asked to do is use this information to calculate the
2:51concentration of h2so4
2:53in the original ten milliliters sample
2:57before we start the calculations for the staycation we need to balance formula
3:01equation for the neutralization a beach to SF for a biennial wage
3:06will write the equation here
3:08to start any type a tight range in calculation the first question we ask
3:12ourselves is
3:13which reacting do we have enough information to find the malls are
3:17in this case is it Neoh her h2so4
3:22worried the first sentence carefully the following the .5 mall there any wage
3:26needed to neutralize ten milliliters to be h2so4
3:30his point 0131 leaders
3:34this does this mean oh the concentration and the volume
3:37up the NaOH solution knowing both its concentration
3:40and volume enables us to find the malls have any wage
3:45the only thing we know about the h2so4 solution at this point is the volume
3:50so we do not have enough information to find the malls and h2so4 yet
3:55so we know that the reactant we can find the malls are is any wage
4:01so now will create a plan to his problem we have the concentration and the volume
4:05a penny a wage
4:06so we start with those
4:09and use them to calculate the malls are many wage
4:12we then use the coefficients in the balance neutralization equation
4:17to calculate the malls and h2so4
4:20in the last step we use the malls h2so4
4:23and the volume up the solution to calculate the concentration of h2so4
4:28in the solution
4:30the concentration of h2so4 can be represented
4:33either as see h2so4
4:37or h2so4 in square brackets
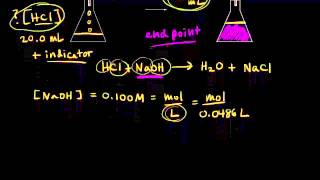
4:40the first step in the calculation is to calculate the malls have any weight
4:45malls is equal to concentration times volume
4:48the concentration have any wages .5 molar
4:51or .5 miles per year
4:54we multiply that by the volume a venue age which is point 0131 leaders
4:59the leaders will cancel and the answer comes out 2.00 655 moles of NaOH
5:06the next step is to calculate the mall zip h2so4
5:09they were neutralized by point 0065 malls have any wage
5:14to do this we used to call fishing ratio and the balanced equation










![[3] Calculations in Titrations](https://i.ytimg.com/vi/3y6BcDmHX5U/mqdefault.jpg)

















