What Are Half Equations | Reactions | Chemistry | FuseSchool
In this video, we will learn how to write half equations for simple redox reactions.
A halfequation shows what happens at one of the electrodes during electrolysis, so we need to be able to complete and balance halfequations for these reactions.
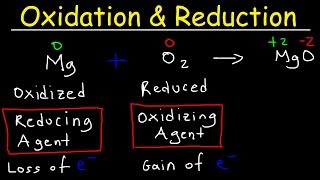
Remember that oxidation occurs at the anode, and reduction occurs at the cathode. Oxidation means the loss of electrons and reduction means the gain of electrons.
Electron transfer has to happen, with one substance losing electrons and another substance gaining electrons.
Half equations take out half of the reaction so looking just at the loss or gain of electrons. We use a copper and silver example in this video to show the half equations.
Each equation represents HALF of a REDOX reaction either the reduction reaction or the oxidation reaction. When the two equations are added together to form the final redox reaction, you can see everything: the number of electrons and are balanced.
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easytounderstand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at www.fuseschool.org, where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
• Complete & Incomplete Combustion | En...
Find all of our Biology videos here:
• The Lymphatic System | Physiology | B...
Find all of our Maths videos here:
• Video
Twitter: / fuseschool
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us: / fuseschool
Friend us: / fuseschool
This Open Educational Resource is free of charge, under a Creative Commons License: AttributionNonCommercial CC BYNC ( View License Deed: http://creativecommons.org/licenses/b... ). You are allowed to download the video for nonprofit, educational use. If you would like to modify the video, please contact us: [email protected]