Write note on Photometric Titrations. | Spectroscopy | Analytical Chemistry
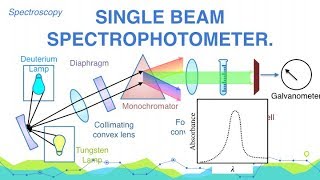
Those titration in which absorbance of the solution is used to determine the end point are called photometric titrations. The method is based on the fact that the absorbance of the solution is directly proportional to concentration. During the course of titration the concentration of the solution being titrated changes. So the absorbance of the solution changes. The end point is obtained from the graph by plotting Absorbance Vs volume of titrant added.
The photometric titrations are of the following types:
1. If titrand is the absorber while titrant and products do not absorb then O.D. decreases during the titration and remain constant after the end point is reached. e.g. In the Estimation of Fe(III)ions with EDTA first Fe (III) are allowed to combine with salicylic acid forming ferric salicylate which is deep colored complex with λmax = 525nm. This complex is titrand and the titrant is EDTA. Titration is carried out at pH = 2.4. Under these conditions EDTA iron complex is much more stable than the iron salicylic acid complex. During the titration the colour will slowly disappear as the end point is reached.
2. If the titrant is capable to absorb then O.D. initially remain constant but increases once the end point is reached as excess titrat is added. e.g. titration of ASCl3 with Brominating mixture (KBr + KBrO3)
3. When the products are capable to absorb the radiations while titrand and titran cannot absorb. Thus as the titrant is added to titrand the product formation take place, so the absorbance increases up to the equivalence point and after that it remain constant. e.g. titration of Cu(II) or Ni(II) with EDTA at the wavelength of 745nm. The pH of the sol must be 2.4.
4. When the titrant and titrand are capable to absorb and the products cannot, initially absorbance decreases as titrand
diminishes and after the equivalence point, again the absorbance increases due to presence of excess of titrant. e.g. in titration of Red dye with Liquid Br, Red dye is titrand and liquid Br is titrant. When the titration is carried out the colour of the red dye diminishes and becomes colourless, Volume of Titrant so the absorbance decreases and after the equivalence point, again the absorbance increases due to presence of excess of titrant.
5) With the help of this we can also titrate the mixture. Thus, if the mixture of Bi and Cu ions is titrated at the wavelength of 745nm with EDTA give separate and well defined equivalence point by photometric technique as shown in the graph.
Analytical Reasoning
• Solve Problems Related To Ages With T...
English Grammar
• Making Sense With Tense #1 English ...
Interview Skills
• Questions You May Be Asked During an ...
Managerial Economics
• MCQ #1 of Managerial Economics
Royalty Free Stock Footage
• Flowers Royalty free footage
Chemical Thermodynamics Physical Chemistry
• State and Explain first law of thermo...
Ionic Equilibria Physical Chemistry
• Derive expression for Ostwald’s Dilut...
Electrochemistry Physical Chemistry
• What are different types of Reversibl...
Solid State Physical Chemistry
• Explain the following terms | Solid S...
Gaseous State Physical Chemistry
• Postulates of Kinetic Molecular Theor...
Colloidal States Physical Chemistry
• What is Colloidal Solution? | Colloid...
Stereochemistry Organic Chemistry
• Explain Configuration and Conformatio...
Nanomaterials Engineering Chemistry
• Compare top down with bottom up Proce...
Water and Its Treatment Engineering Chemistry
• Explain why hard water gives out a cu...
Electrochemistry Engineering Chemistry
• Distinguish between metallic and elec...
Environmental Studies
• MCQ on Environmental Studies Part 8
Optics Applied Physics
• What are cartesian sign conventions f...
For Details Visit
http://cepekmedia.co.nf
http://cepek.hol.es/
http://edmerls.66Ghz.com/
http://edmerls.tk/